Abstract
Introduction Myelofibrosis (MF) is the most aggressive form of Philadelphia negative myeloproliferative neoplasm and allogeneic hematopoietic stem cell transplantation (HSTC) is the only curative approach, but non relapse mortality limits the indication for HSTC to high-risk patients. The prognostic evaluation of MF patients is evolving during time together with a better understanding of the mutational landscape of this disease. The presence of high molecular risk (HMR) mutations, specifically ASXL1, SRSF2, EZH2, IDH1, IDH2 and U2AF1Q157, harbors a detrimental effect on survival and leukemic transformation and has been incorporated in different prognostic scoring systems. In this multicenter study we aimed to determine the impact of HMR mutations on transplant outcome.
Methods We studied two independent cohorts of patients: 44 patients (training, retrospective cohort) were part of a multicenter, randomized GITMO (Gruppo Italiano Trapianti di Midollo Osseo) clinical trial (Patriarca F, BBMT 2019) and 29 patients (validation, prospective cohort) were subsequently recruited at the Bergamo Bone Marrow Transplant Unit. Patients from the training cohort (median age 56, range 36-66) were conditioned with busulfan-fludarabine or thiotepa-fludarabin while patients in the validation cohort (median age 61, range 36-67) were conditioned with a reduced intensity thiotepa-busulfan-fludarabin regimen. The mutational profile obtained on the pre-transplant DNA was performed by sequencing 30 myeloid related genes by applying SOPHia GENETICS Myeloid Solution on Illumina MiniSeq platform.
Results In the training retrospective cohort, driver mutations were detected in 40 (29 JAK2/8 CALR/3 MPL) patients, with 4 patients having a triple negative MF. At least one HMR mutation was detected in 43% of patients and an unfavorable karyotype was present in 18% of patients. According to the Mutation-Enhanced International Prognostic Scoring System 70 plus (MIPPS70+) scoring system, 77% of patients were allocated in the high/very high groups. With a median follow up of 4.2 years (range 0.02-9), the 5-years Overall Survival (OS) and Progression Free Survival (PFS) were 61% and 45% respectively. The 5-years Non-Relapse Mortality (NRM) and Cumulative Incidence of Relapse (CIR) were 25% and 30%. In the prospective validation cohort, a driver mutation was detected in all patients (15 JAK2/7 CALR/7MPL). At least one HMR mutation was detected in 48% of patients and an unfavorable karyotype was present in 24% of patients. Patients allocated to the MIPPS70+ high/very group were 52%. After a median follow up of 10.7 months (range 0-44), the 2-years OS and PFS were 61% and 49% respectively. The 2-years NRM and CIR were 33% and 18%.
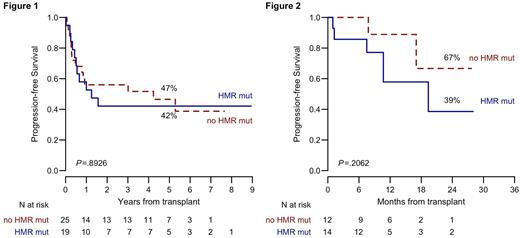
In the training cohort, a higher risk of death and progression was observed in patients with high/very high risk MIPPS70+ prognostic index [HR for OS 4.3 (95% CI 0.6-33.1), HR for PFS 4.1 (95% CI 0.9-16.9)]. Conversely, the presence of HMR mutations did not significantly affect OS, PFS (Figure 1), NRM or CIR. The prognostic significance of MIPSS70+ risk classification was confirmed in the validation cohort, with a higher risk of death and progression for high/very high MIPSS70+ group [OS HR 3.2 (95% CI 0.4-27.1), PFS HR 3.8 (95% CI 0.5-31.4)]. Similarly, in the validation cohort the presence of HMR mutations did not affect transplant outcome, with no impact on OS, PFS (Figure 2), NRM or CIR. In the whole group of 73 patients, the presence of JAK2V617F mutation showed an adverse prognostic impact on CIR [HR 3.4 (95% CI 1-11.8)], while MIPPS70+ confirmed its role on PFS.
Conclusions In this study we prospectively confirmed that the presence of HMR mutations did not impact on transplant outcome. Allogeneic stem cell transplantation can lead to a significant cure rate of MF patients, regardless the presence of HMR mutations. Patients in the high/very high MIPSS70+ risk group had a higher risk of death and progression after transplant. Appropriate timing and selection of patients is crucial for transplant outcome in MF.
Disclosures
Patriarca:Amgen: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Guglielmelli:Novartis, Abbvie: Other: Other member of advisory board, speaker at meeting. Luppi:Gilead sci: Other: Travel grant; Abbvie, Jazz Pharma, Gilead sci, MSD, Novartis, Sanofi, Daiichi-Sankyo, Grifols: Membership on an entity's Board of Directors or advisory committees. Polverelli:Novartis Pharma: Consultancy. Rambaldi:Janssen: Honoraria; Roche: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Celgene-BMS: Honoraria; Omeros: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal